How Many Hydrogen Bonds Can Urea Form
The oxygen atoms of the CO groups each have two unshared pairs of electron and can form two hydrogen bonds with hydrogen atoms for a total of four hydrogen bonds. It has a role as a flour treatment agent a human metabolite a Daphnia magna metabolite a Saccharomyces cerevisiae metabolite an Escherichia coli metabolite a mouse metabolite and a fertilizer.

Solvation Structure Of The Urea Molecule From Our Experiments One Of Download Scientific Diagram
In the case of DNA the new strand becomes part of a stable helix.
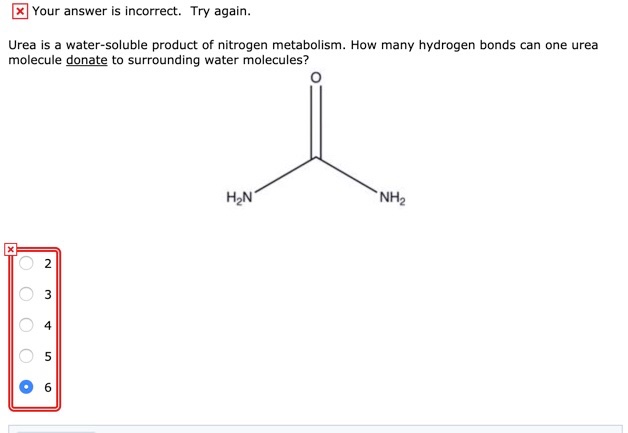
. Therefore an urea molecule has three atoms that can act as hydrogen bond acceptors and four atoms that can act as hydrogen bond donors. The commercially available fertilizer has an analysis of 46-0-0 N-P2O5-K2O. To form two molecules of ammonia NH3 and one.
Although urea has nearly three times the molecular volume of water the structure of liquid water is sufficiently open that a urea molecule appears to displace just two waters offering up to eight hydrogen bonds in place of the displaced pair. Since this is all hydrogen needs it can only form one bond. Instead there will be a good number of associations by hydrogen bonds between water molecules urea molecules.
Q042 Both DNA and RNA are synthesized by covalently linking a nucleoside triphosphate to the previous nucleotide constantly adding to a growing chain. Urea is a carbonyl group with two C-bound amine groups. Experts are tested by Chegg as specialists in their subject area.
The answer is 6. The two strands are complementary in sequence and antiparallel in directionality. This problem has been solved.
You are right that the four hydrogens connected to the nitrogens could create a hydrogen bond with water. Thus the maximum number of water molecules that can connect to urea is 8. Urea is a soluble compound and easily gets along with water.
View the full answer. However additionally the two nitrogens each have one set of lone pairs and the oxygen molecule has two lone pairs open for hydrogen bonding. Question 33 1 pts Q026 Larger molecules have hydrogen-bonding networks that contribute to specific high-affinity binding.
Who are the experts. Finally the oxygen in the urea molecule may form hydrogen bonds with water as well but it has two lone pairs to donate so the oxygen atom may form hydrogen bond with 2 water molecules. The other hydrogen bond O 9 H 4 N 2 is slightly longer rOH 2067 Å.
Density functional theory DFT calculations are performed to study the conformations hydrogen-bonding network and the stabilities of all possible molecular associations ureaH 2 O n n 15 in aqueous solutions of urea. I hope this helps. So for each nitrogen in urea there would be a hydrogen bond and for each hydrogen too.
This makes it very useful to cap of different lose ends in molecules. Carbonic acid H2CO3 via the. The B3LYP functional and the basis set 6-311Gd p are used through the calculations.
The intermolecular bonds are hydrogen bonds. The chemical structure of urea helps it to be soluble enough as the hydrogen bonds with water molecules each forming two bonds with oxygen. Due to the strong hydrogen bonding the average NH.
N2 because of the oxygen atom of the urea4 because of the hydrogen. The water molecule is H2O. Hydrogens electronegativity is also relatively average 224 for nonmetals and metals which means that it normally forms both polar and nonpolar covalent bonds.
One of the water hydrogen atom interacts with the urea oxygen atom through hydrogen bonding H 10 O 9 rOH 1876 Å. In crystalline urea the oxygen atom participates in four hydrogen bonds 52. Playlist Organic Chemistry questions for practice.
What is the maximum number of water molecules that one urea molecule can hydrogen bond with. In the framework of synthesizing amides amines and urea from abundant building blocks and renewable electricity CN-bond-forming reactions would entail the use of CO 2 and N 2 NO 3 NO 2. Each bond allows for two electrons to be shared.
Use a funnel to pour the solution into the sprayer shake well. The vibrational spectra of urea also demonstrate such effects. When two atoms form polar covalent bonds the atom that ends up with the greater share of electrons is said to be oxidized while the.
In general mix 1 heaping tablespoon of a 46-0-0 urea fertilizer in 1 gallon of water to make a 05 percent solution or use 4 tablespoons of fertilizer to make a 2 percent solution. How many hydrogen bonds can urea form. In this model the urea molecule is linked by one N HO and one O HO hydrogen bonds to water molecule Fig.
Theoretically there are a maximum of 5 water molecules that one urea molecule can hydrogen bond with but there are 6 hydrogen. Larger molecules have hydrogen bonding networks that contribute to specific high affinity bonding smaller molecules such as urea can also form these networks how many hydrogen bonds can urea form in water. The planar structure further enhances.
See the answer See the answer done loading. We review their content and use your feedback to keep the quality high. Urea has an oxygen atom attached to the main carbon that can act a.

Solvation Structure Of The Urea Molecule From Our Experiments One Of Download Scientific Diagram
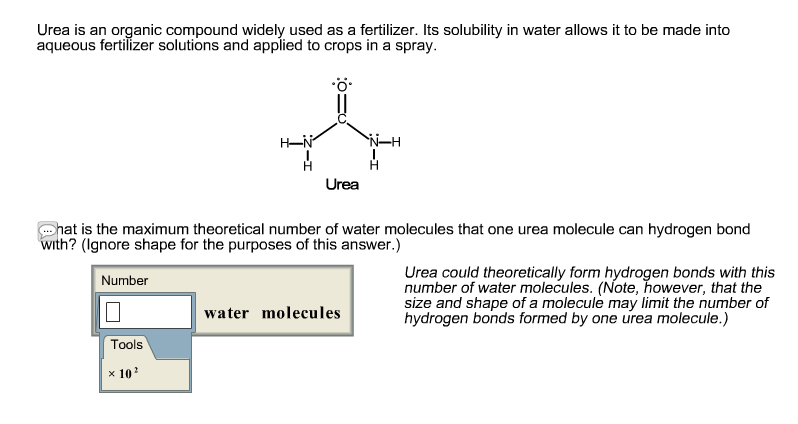
Solved Urea Is An Organic Compound Widely Used As A Chegg Com

Solved X Your Answer Is Incorrect Try Again Urea Is A Chegg Com
No comments for "How Many Hydrogen Bonds Can Urea Form"
Post a Comment