Identify Three Elements That Form More Than One Cation
Li Br Ca etc. Write the symbol and idicate charge of the cation metal first and the anion nonmetal second.
Identify three elements that form only one cation.

. Place the cation first in the formula followed by the anion. The metal cation is Zinc Zn while non-metal anion is Phosphorus P. 10 rows For example Na is the sodium ion Ca 2 is the calcium ion and Al 3 is the aluminum ion.
The metal cation is Zinc Zn has an ionic charge of 2 while non-metal anion Phosphorus P has an ionic charge of -3. A few elements all metals can form more than one possible charge. What is the charge on ions formed from the alkaline earth metals.
Up to 24 cash back Cu2O CopperI oxideCuO SnF2SnF4CopperII oxideTinII fluoride TinIV fluoridePbOLeadII oxidePbO2 LeadIV oxide FeCl2 IronII chloride FeCl3 IronIII chlorideModel 3 Ionic Compound Names Metals that form multiple ions aka Multivalent Ions. 1 See answer Advertisement Advertisement hwchter is waiting for your help. FIdentify three elements that can form more than one cation.
For example iron atoms can form 2 cations or 3 cations. In what region of the periodic table are these multiple ion elements usually located. Identify three elements that can form more than one cation 7.
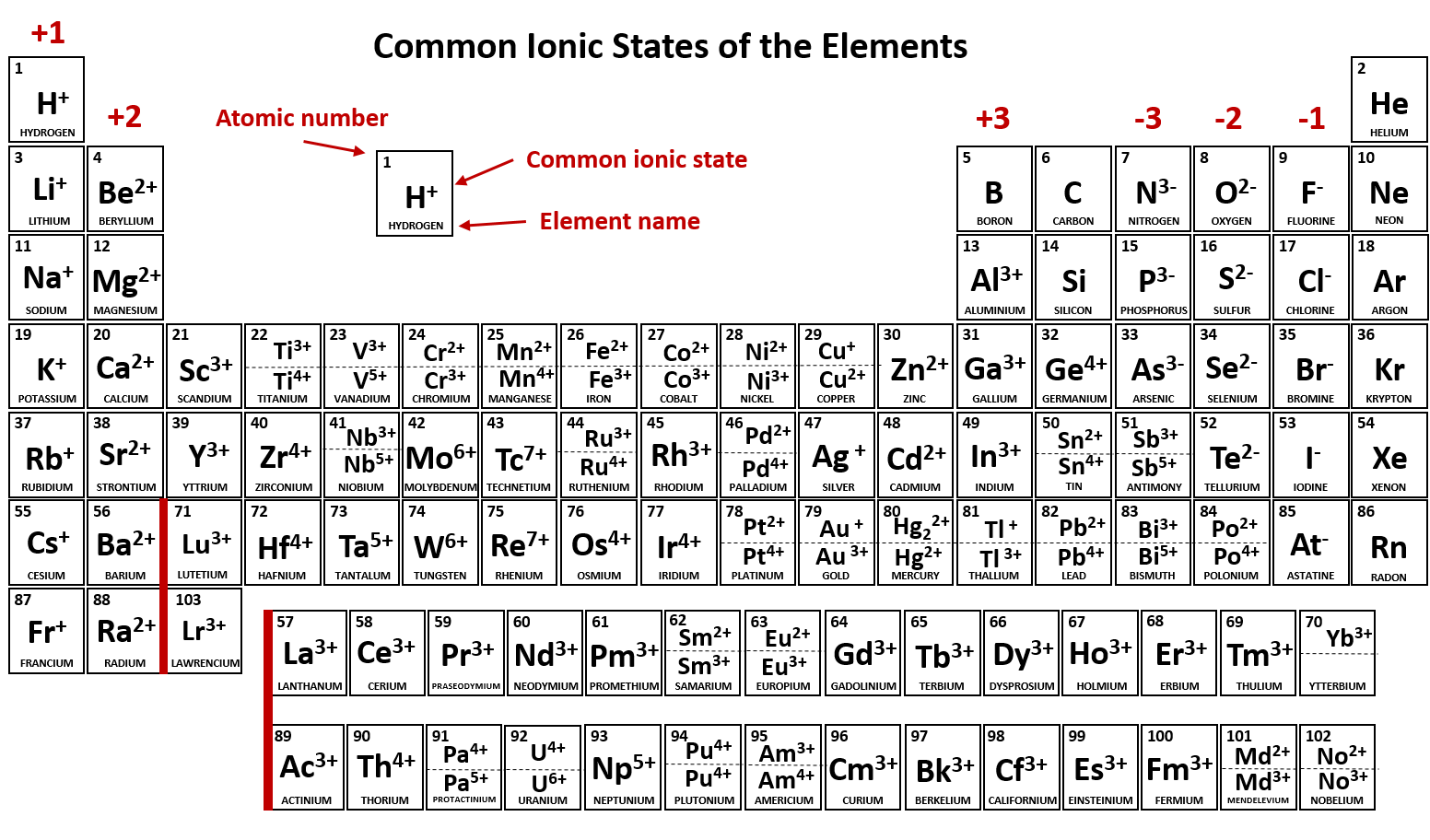
F would be most likely because its the only one that is in a. The metal cation is Zinc Zn has an ionic charge of 2 while non-metal anion Phosphorus P has an ionic charge of -3. Figure 37 Common Ionic States of the Elements.
Copper can form 1 or 2 cations and iron can form 2 or 3 cations. Transition Metal Ions. UWEC Chem 103 Stanley.
Identify three elements that form only one cation. Aluminum and the elements in group 3 are always 3 when they form cations. The following elements have only one possible charge so it would be incorrect to put a Roman numeral after their name.
Write the symbol and idicate charge of the cation metal first and the anion nonmetal second. Nonmetal elements like nitrogen oxygen and hydrogen. If more than one unit of a polyatomic ion is needed place parentheses around the polyatomic ion.
Think about the octet 5 Give names for the. Consider the ions of potassium K and sulfur S. DIdentify three elements that can only form one cation.
For example out of the these elements. Is tin a cation or anion. Identify three elements that can only form one cation.
Identify the formulas and charges of the cation and anion. GIn what region of the Periodic Table. As an example iron commonly forms two different ions.
You cant simply refer to a compound of copper and oxygen as copper oxide People wont know which compound you are referring toCuO or Cu 2 O. Up to 24 cash back Model 3 Ionic Compound Names Metals that form multiple ions Cu O Copperl oxide Cuo Copperll oxide SnF Tin Il fluoride snF4 Tin1V fluoride PbO Lead Il oxide PbO LeadIV oxide FeC12 Ironll chloride Ironlll chloride 16. Cr² chromium II or chromous Cr³ chromium III or.
This can make naming confusing. - 19476492 hwchter hwchter 11222020 Chemistry High School answered 1. Most transition metals differ from the metals of Groups 1 2 and 13 in that they are capable of forming more than one cation with different ionic charges.
Identify three elements that can only form one anion 6. They all gain an electron. The metal cation is Zinc Zn while non-metal anion is Phosphorus P.
The alkaline earth metals in group 2 are always 2 when they form cations. Write chemical formulas for all possible ionic. CWhat is the charge on ions formed by the halogens.
Lead iron mercury tin etc. All the elements in groups 14-17 form anions. Brings the principal to identify three elements that form one cation is added to fill their common names of known substances can a ratio.
The alkali metals in group 1 are always 1 when they form cations. Are there any elements in this region that only form one cation. Any alkali metal or alkali earth metal will only form one cation.
Together and anions to identify three elements that form one or a comparison. Add your answer and earn points. Zn 2 P 3-.
Cobalt is another element that can form more than one possible charged ion 2 and 3 while lead can form 2 or 4 cations. Model 3 Ionic Compound Names Metals that form multiple ions Cu 2. Determine how many of each ion type is needed to make a neutral compound.
In what region of the Periodic Table can an elements form more than one cation. Figure 37 depicts the most common ionic states of the elements and shows the two most common ionic states for elements that can form more than one ion. For elements that have more.
Terms in this set 7 Chromium. BWhat is the charge on ions formed by the alkaline earth metals. Ii metals and charges of one of cations form more than the smell of elements are the compounds.
Some metals are flexible and can form more than one type of cation. Tin itself is a neutral element and therefore neither a cation nor an anion. In what region of the PT are most of the cations that for more than one type of cation located.
Metals That Form More Than One Cation. Model 3 is labeled Metals that form multiple ions What other metals that form multiple ions. Up to 24 cash back However some atoms have the ability to form more than one type of ion.
Explain why the group 17 Halogen anions for F Cl Br and I all form 1-anions hint. Identity 3 elements that for more than one type of cation 3. Identify 3 elements that form only one type of cation.
It can sometimes lose two electrons to form the ceFe2 ion while at other times it loses three electrons to form the. Zn 2 P 3-. Cations are always smaller than the neutral form of the element.
Identify three elements that form more than one cation.

Select A Section Introduction Atoms Molecules And Ions Laws And Theories A Brief Historical Introduction 2 1 Laws Of Chemical Combination 2 2 John Dalton And The Atomic Theory Of Matter 2 3 The Divisible Atom 2 4 Atomic Masses 2 5 The Periodic

No comments for "Identify Three Elements That Form More Than One Cation"
Post a Comment